Research article | Published November 25, 2022 | Original research published in Nature Methods
Real-Time, Multicolor Fluorescence Imaging of Tumors in Tissues
Jay Freeman
Jay Freeman is a multidisciplinary chemist with post-doctoral experience in pharmaceutical and medicinal chemistry. He is currently a science editor and writer with Oxford Science Editing.
Original research: Bandi, V.G., Luciano, M.P., Saccomano, M. et al. Targeted multicolor in vivo imaging over 1,000 nm enabled by nonamethine cyanines. Nat Methods 19, 353–358 (2022). https://doi.org/10.1038/s41592-022-01394-6
Subjects: Cancer imaging, Chemical tools
New Ground article reviewed by: Oliver T. Bruns
In vivo fluorescence imaging is a powerful, non-invasive technique for observing biological processes, structures and disease in living organisms. It is based on the phenomenon of fluorescence, whereby a molecule absorbs light energy of a certain wavelength and briefly enters an excited state before returning to its normal state, releasing the absorbed energy as light. However, because not all the absorbed energy is released as light – some is spent on non-radiative transitions such as molecular vibration – the emitted light is characterized by slightly lower energy (and thus a slightly higher wavelength) than the incident light, which allows it to be discriminated by a sensitive detector. Molecules that exhibit this behavior are called “fluorophores,” and the wavelengths at which they absorb and emit light depend on their chemical structures.
A recent paper by Venu G. Bandi and an international team thoroughly exemplifies the modern, multidisciplinary approach to cutting-edge research in the field of in vivo fluorescence imaging. It represents a significant and potentially far-reaching breakthrough that provides novel fluorophores and enables higher-resolution imaging of deeper tissues, as well as simultaneous, multicolor imaging of multiple tissue types. Implemented clinically, these developments promise to improve therapeutic outcomes and save lives.
Fluorophores in practice
For practical purposes, fluorophores are usually linked to biomolecules such as proteins or antibodies that have an affinity for particular tissues, organs or biostructures. This linking process, known as “bioconjugation,” endows a fluorophore with the ability to aggregate at a specific site after injection into the body, providing a means for observing the target tissue. A common example here is the bioconjugation of a fluorophore to an antibody with an affinity for tumors.
Fluorophores used for in vivo imaging must also be water-soluble, or they will not be transported to the target area by the blood plasma; they must be somewhat stable in vivo, or they will break down or be metabolized before reaching the target area; and they must not be toxic. Taken together, these factors are referred to as “biocompatibility.”
Moreover, for a fluorophore to be of practical use, the incident light beam must have sufficient tissue-penetrating ability to reach the fluorophore at the target site within the body and excite it, and the light emitted by the fluorophore must have sufficient tissue-penetrating ability to escape to the outside world with sufficient intensity to be detected. Visible light has very poor tissue-penetrating ability (as illustrated by the fact that only major veins and arteries beneath the skin are visible to the naked eye).
Light in the wavelength range 700 to 1000 nanometers (nm), or near-infrared (NIR) range, exhibits much better tissue penetration and is currently widely employed in fluorescence imaging, but this methodology is limited by light scattering and the capabilities of the silicon-based devices used to detect the NIR emission. However, recent studies have shown that light in the 1000 to 2000 nm range, or shortwave-infrared (SWIR) light, allows fluorescence imaging at resolutions and tissue depths far superior to those attainable using NIR detection. It is the SWIR range that is exploited by the advances in the presented work.
Collectively then, an effective in vivo fluorescence imaging system can be thought of as comprising three elements: a sufficiently tissue-penetrating light source; a biocompatible fluorophore that absorbs light at the wavelength supplied by the light source; and a detector that is compatible with the fluorophore’s emission wavelength. Accordingly, the work of Bandi’s team can be viewed as a response to advances in SWIR detection technology, specifically, the increased availability of indium-gallium-arsenide (InGaAs)-based SWIR detectors, which provide sensitive detection in the 1000 to 1700 nm range. To quote the study directly, “As InGaAs detector-based imaging systems have become more readily available, a critical bottleneck in this field is access to biologically compatible fluorescent probes that operate in this range.”
FNIR-1072: A novel SWIR fluorophore
Indocyanines (Fig. 1) are commonly used in fluorescence microscopy, and their derivatives are the most widely applied fluorophores in NIR fluorescence imaging, both clinically and in laboratory research. Indocyanine green (Fig. 1a), a heptamethine fluorophore, has numerous medical applications and emits light in both the NIR and SWIR range. Cyanines bearing certain organic heterocycles – ring structures with at least two different chemical elements – have been reported to exhibit excitation and emission in the SWIR range. Such behavior, however, has not yet been demonstrated for bioconjugated cyanines. This aspect, coupled with the research team’s experience in tuning methine scaffolds (for examples, see Fig. 1), led them to explore nonamethine indocyanines, the goal being to develop novel, targetable SWIR fluorophores.
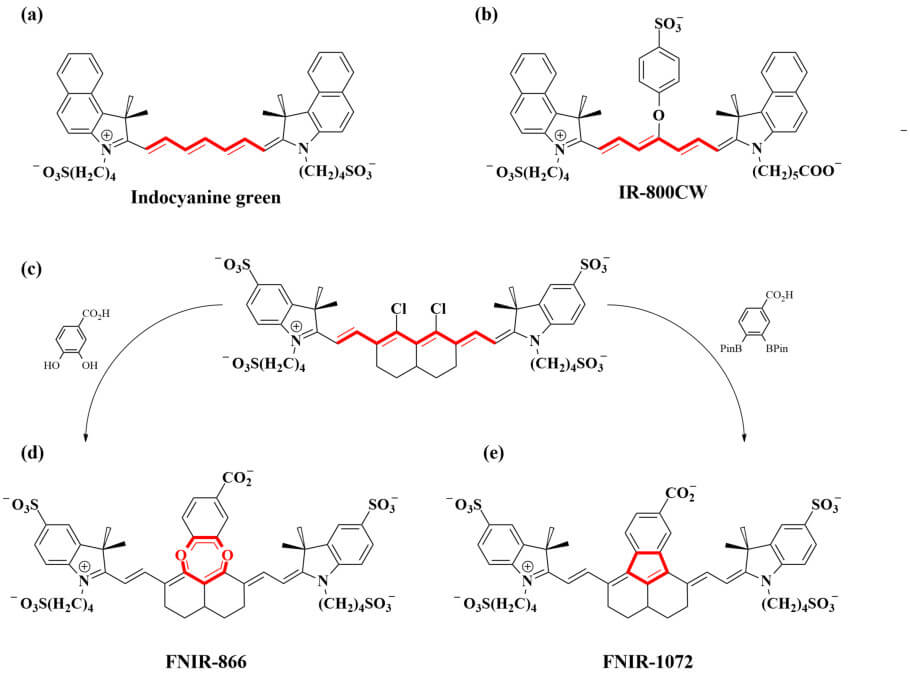
A key aspect of their work is the use of density functional theory to facilitate the rational design of the fluorophores. This powerful methodology computationally simulates molecules and chemical systems, allowing their properties and interactions to be predicted. In the first instance, the team used density functional theory to explore the effects of different substituents on the nonamethine scaffold. Their results indicated that the presence of a fused catechol moiety (illustrated in Fig. 1d) would provide a fluorophore with absorbance/emission behavior typical of cyanines that absorb light near 1000 nm, and that a similar structure without the catechol linkage but instead with direct linkage of the aryl moiety to the central structure (illustrated in Fig. 1e) would absorb and emit light at >1000 nm, i.e., in the SWIR range.
Accordingly, persulfonated versions of these two fluorophore structures, designated FNIR-866 (Fig. 1d) and FNIR-1072 (Fig. 1e) respectively, were prepared by means of an elegant “divergent synthesis,” i.e., one that involves the derivatization of a single intermediate to generate numerous product compounds (Fig. 1c). In this , “FNIR” stands for “Frederick near infrared” and refers to the location (Frederick, Maryland) of the US National Cancer Institute/National Institutes of Health laboratory in which this series of dyes was developed; the numbers represent the molecules’ real (rather than predicted) absorbance wavelength maxima.
The physicochemical and photophysical properties of FNIR-866 and FNIR-1072 were investigated, revealing that they have stabilities and solubilities comparable to those of the heptamethine cyanine NIR-fluorescent probe IR-800CW (Fig. 1b), which is already clinically used for in vivo imaging.
FNIR-872 (a modified version of FNIR-866, as the latter proved unamenable to bioconjugation) and FNIR-1072 were then bioconjugated to the monoclonal antibody panitumumab (Pan), which targets tumor cells, yielding the bioconjugates FNIR-872-Pan and FNIR-1072-Pan. The clinically proven probe IR-800CW-Pan was used for comparison. The researchers injected these three probes separately into mice bearing implanted subcutaneous tumors. The two novel conjugates exhibited similar tumor-targeting abilities, tumor-to-background signal ratios, and biodistributions to those of IR-800CW-Pan, clearly demonstrating their potential clinical utility. Most importantly, when probe FNIR-1072-Pan was excited by SWIR light, an InGaAs detector was able to record the emission of SWIR light.
Multiplexing: Simultaneous detection of different tissues
At this stage it is necessary to introduce a further level of complexity to the discussion of in vivo fluorescence imaging. Imagine a situation in which multiple bioconjugated fluorophores are injected simultaneously into a human or animal, and that these probes have affinities for different tissue types and emit light at different wavelengths. This would allow distinct tissues to be represented by different colors of fluorescence simultaneously, an approach that is called “multiplexing”. In the in vivo multiplexing work done by Bandi and colleagues, all the detection took place in the SWIR range, with the excitation-light wavelength for the probes being either in the NIR or the SWIR range, depending on the absorbance maximum of the particular probe used.
The team explored the use of FNIR-872-Pan along with a dextran conjugate of another fluorophore, FNIR-Tag, called FNIR-Tag-Dex, in multiplexing (FNIR-Tag is a heptamethine cyanine fluorophore developed in a previous study that also involved some of the authors of the paper presented here). Bioconjugates of dextran, which is a complex branched polysaccharide, are often used to probe metabolic processes. In these two-component multiplex experiments, FNIR-Tag-Dex was accumulated and excreted by the kidneys, while FNIR-872-Pan targeted the tumor. Thus, the combined administration of FNIR-Tag-Dex and FNIR-872-Pan enabled the simultaneous color-contrasted visualization of kidney tissue and a subcutaneous tumor. Accordingly, this experiment verified not only the probes’ multiplexing capabilities, but also their utility in visualizing deep-seated tissue such as kidney tissue.
Encouraged by these results, the team then showed that multicolored SWIR imaging using FNIR-872-Dex (i.e., a bioconjugate of FNIR-872 and dextran) administered in combination with indocyanine green allowed a deep-seated liver tumor to be detected in mice and to be distinguished from surrounding normal (healthy) tissues. When applied systemically, indocyanine green is cleared from healthy liver tissue into the intestine and excreted, but it marks liver tumors, which remain stained after the clearance. In this work, indocyanine green was administered first and, as expected, left the tumor stained after being excreted by the healthy liver tissue. Then, FNIR-872-Dex was administered, which accumulated in macrophages, a type of cell that is present in healthy liver tissue but not in tumor tissue. Thus, the indocyanine green provided a signal for the tumor tissue, while the probe FNIR-872-Dex specifically enhanced the surrounding healthy tissue. In combination, the two agents provide a very clear contrast between the tumor and the normal tissue.
Superiority of SWIR to NIR
In a healthy person, the lymphatic system is important for fighting infection and destroying old or abnormal cells. Lymphatic pathways run throughout the body and meet at lymph nodes, which contain the functional parts of the system. However, in cancer patients, tumor cells can spread (metastasize) throughout the body and reach other organs via the lymphatic system. The first lymph node to which cancer cells are likely to spread from a primary tumor is known as the sentinel lymph node. Hence, the lymphatic system is crucial in the development, monitoring and treatment of cancer.
As a continuation of their multiplexing experiments, the researchers labeled a tumor with FNIR-872-Pan and subsequently injected indocyanine green into the tissue near the tumor to discriminate the lymphatic vessels (when indocyanine green is injected into tissue, it is subsequently transported away via the lymphatic pathway; in this way, it marks the lymphatics and the sentinel lymph node).
Finally, the use of FNIR-1072-Dex in combination with indocyanine green and FNIR-872-Pan was demonstrated to enable tumor labelling (by FNIR-872-Pan), with simultaneous visualization of the blood vessels in the surgical field (by FNIR-1072-Dex) and the lymphatic vessels and sentinel lymph node (by indocyanine green).
Thus, the simultaneous use of these probes made it possible to clearly identify the distinct key tissue structures for tumor surgery, the tumor and the remaining margins in the tissue, as well as the sentinel lymph node, which potentially contains metastatic tumor cells.
Outlook
The work of Venu G. Bandi and his team, overseen by Oliver T. Bruns and Martin J. Schnermann, presents a powerful and inspiring combination of such diverse fields as physical chemistry, organic synthesis, computational chemistry, physics, engineering, biology and oncology. Their experiments amply demonstrate the utility of their novel probes in real-time multiplex in vivo imaging. Clearly, such imaging is of major value to surgeons and clinicians and, should clinical assessment of the team’s probes prove successful, holds enormous potential.
Its scope of application may be extended even further if the authors’ future work, which will explore the possibility of making the probes stimulus-responsive, also bears fruit. For example, a responsive probe could enable the visualization of neuronal activity by imaging calcium waves.
How to reuse
The CC BY 4.0 license requires re-users to give due credit to the creator. It allows re-users to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.
You can reuse an article (e.g. by copying it to your news site) by adding the following line:
Multicolor Fluorescence Imaging of Tumors in Tissue © 2022 by Jay Freeman is licensed under Attribution 4.0 International
Or by simply adding:
Article © 2022 by Jay Freeman / CC BY
To learn more about the available options, and for details, please consult New Ground’s How to reuse section.
This article – but not the graphics or images – is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
This article – but not the graphics or images – is licensed under a Creative Commons Attribution 4.0 License.
