Research article | Published June 9, 2023 | Original research published in Nature Cardiovascular Research
Single-Cell Analysis Supports a Role for T cell Autoimmunity in Atherosclerosis and Helps Pave the Way for New Immunotherapies
Katrina Woolcock
Katrina Woolcock is a Scientific Editor & Writer at Life Science Editors (website). She obtained her Ph.D. in Molecular Biology from the Friedrich Miescher Institute for Biomedical Research in Basel, Switzerland, and completed a locum position as an editor at Nature Structural & Molecular Biology.
Original research: Wang, Z., Zhang, X., Lu, S. et al. Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis. Nat Cardiovasc Res 2, 290–306 (2023). https://doi.org/10.1038/s44161-023-00218-w
Subjects: Atherosclerosis, Autoimmunity, T cells
New Ground article reviewed by: Andreas J. R. Habenicht
T cells, an important component of the adaptive immune system, recognize peptides presented by antigen-presenting cells, e.g., dendritic cells and macrophages, by their T cell receptors. This recognition event triggers T cell activation and proliferation, or clonal expansion, launching an immune response. To ensure “tolerance of self” – i.e., tolerance of the body’s own healthy cells and tissues – and thereby prevent autoimmunity, T cells are regulated through various “tolerance checkpoints.” In certain cases, such as cancer, inhibiting these checkpoints can be therapeutically beneficial, as it boosts the immune response to e.g. tumor cells. However, when tolerance checkpoints break down, autoimmune diseases like multiple sclerosis, type 1 diabetes, and psoriasis can be the result.
T cell responses have also been observed in atherosclerosis, the leading cause of cardiovascular disease. Atherosclerosis begins as a condition in which lipids accumulate in the inner layer of arteries, but it can progress to the formation of atherosclerotic plaques that contain fatty substances, cellular debris, and immune cells including macrophages and T cells. These plaques may eventually become unstable and rupture, leading to local blood clotting that can suddenly block an artery, causing a heart attack or stroke. Until recently, it was unclear where the T cell responses associated with atherosclerosis took place and whether autoimmune T cells were involved.
To address these questions, a team of researchers led by Changjun Yin and Andreas Habenicht together with Zhihua Wang and Christian Weber leveraged recent advances in single-cell technologies. Their study “Pairing of single-cell RNA analysis and T cell antigen receptor profiling indicates breakdown of T cell tolerance checkpoints in atherosclerosis,” published in Nature Cardiovascular Research, shows that tolerance checkpoints are disrupted in atherosclerosis in tissue-specific and T cell-subtype-specific ways and strongly suggests a role for autoimmune T cells in atherosclerosis.
A role for adaptive immunity in atherosclerosis
Initially, it was believed that the inflammation observed in atherosclerosis resulted from activated innate immune responses, which can be mitigated by statin drugs that lower LDL cholesterol and thereby reduce the generation of plaque. However, many individuals still exhibit signs of inflammation despite taking statins. The notion that adaptive immunity, as opposed to innate immune responses, is involved in this residual inflammation emerged in the late 1980s, when it was discovered that human atherosclerotic plaques contained activated T cells. Helper T cells were found to be pro-atherogenic, whereas regulatory T (Treg) cells were found to be anti-atherogenic.
These studies sparked interest in developing immunomodulatory therapies to prevent cardiovascular disease by suppressing pro-atherogenic responses and promoting anti-atherogenic ones. However, our limited understanding of the dynamic changes and crosstalk between heterogeneous immune cell subsets in atherosclerosis has hindered progress in pursuing such therapeutic approaches.
Tackling heterogeneity with single-cell analyses
The past decade has witnessed remarkable advances in single-cell technologies, including single-cell proteomics and single-cell RNA sequencing. These technologies hold the potential to identify new targets, pathways, and immune interactions, facilitating the development of precise immunotherapies. Drawing on them, the team investigated the coordinated activities of heterogeneous immune cells in Apoe-/- mice, a well-established model for studying atherosclerosis. Specifically, the researchers employed single-cell transcriptome sequencing and T cell receptor sequencing – scRNA-seq and scTCR-seq – to examine tolerance checkpoints in atherosclerosis. To determine the locations of immune responses, they analyzed blood, atherosclerotic plaques, local lymph nodes, and artery tertiary lymphoid organs (ATLOs) – aggregates of immune cells that form near plaques in response to inflammation. As controls, they used lymph nodes and blood from wild-type mice.
Breakdown of tolerance checkpoints
The research team discovered evidence of tissue-specific breakdown of multiple tolerance checkpoints in the Apoe-/- mice, with the most pronounced effects observed in plaques, followed by ATLOs and then lymph nodes. Compared to healthy tissue, they found increased or decreased populations of specific immune cells and observed the conversion of Treg cells into inflammation-promoting helper T cells. They also found altered expression of tolerance-related RNA transcripts, and other anomalies like altered antigen presentation, all leading to an aberrant immune response. Importantly, T cell receptor sequencing revealed clonal expansion of three T cell subsets – CD4+ T cells, Treg cells and especially CD8+ T cells – in ATLOs and plaques. Since the mice were kept under sterile conditions, this clonal expansion could only have been triggered by self-antigens, indicating autoimmunity.
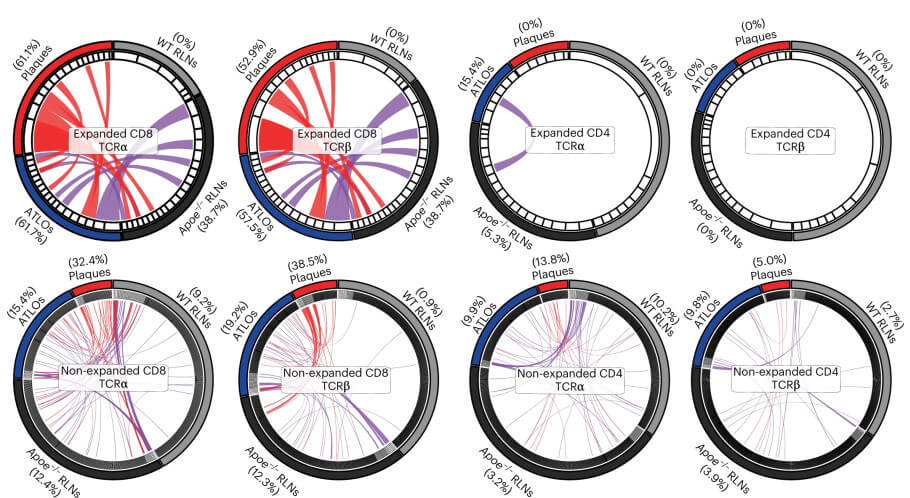
Finally, the team compared their findings with published datasets on human plaques and found that CD8+ T cells and macrophages were similar in terms of their cell numbers and transcript profiles between human and mouse plaques. Therefore, the mechanisms of tolerance breakdown involving these cells may also apply to human atherosclerotic plaques.
Future work will identify the self-antigens
To summarize, the study demonstrates that tolerance checkpoints are compromised at multiple levels in advanced atherosclerosis in mice, particularly within the plaques themselves. Furthermore, plaque-infiltrating T cells likely recognize atherosclerosis-relevant self-antigens, leading to the T cells’ activation. These autoimmune T cells probably contribute to the control of plaque growth.
Interestingly, these findings may also explain why checkpoint inhibitors that have been successful in cancer treatment can actually worsen atherosclerosis. By unleashing T cells with prior antigen exposure, these inhibitors may activate immune responses to atherosclerosis-specific epitopes. Accordingly, identifying the distinct autoimmune T cell responses in both cancer and atherosclerosis will be of considerable interest.
As always, however, the findings have certain limitations. It is important to bear in mind that techniques used to isolate single cells may affect phenotypes so that the analyses might not exactly reflect the original cell state. Furthermore, the Apoe-/- mouse model does not fully reflect the processes in human atherosclerotic disease. Finally, the study did not identify the specific self-antigens recognized by the T cells; however, the identification of three T cell subsets as being clonally expanded in specific tissues paves the way for future investigations into the epitopes involved. For instance, prediction software could identify potential epitopes based on the T cell receptor sequences.
Implications for immunotherapy
The findings hold significant translational potential. Future research will shed light on which tolerance checkpoints affect the progression of atherosclerosis, enabling the design of restorative therapies. In the case of self-antigens that bind to Treg cells, the outcome may be anti-atherogenic. Therefore, engineered autoimmune Treg cells could conceivably be used to dampen the immune response: T cells would be extracted from the patient, modified according to the T cell receptor sequences identified by the authors, expanded into millions, and infused back. By contrast, for self-antigens that bind to CD4+ or CD8+ cells, the outcome may be pro-atherogenic. In this case, tolerogenic vaccines could be developed to induce self-antigen-specific tolerance.
Such therapies would specifically target plaque inflammation, as opposed to using systemic anti-inflammatory treatments, which are associated with an increased risk of infection. However, immunotherapies may need to be tailored to specific patient groups based on their clinical status. In this regard, single-cell technologies like those used by Zhihua Wang et al. will play a vital role in guiding the design of future personalized immunotherapies.
In conclusion, the researchers have provided a rationale for future immunotherapies aimed at preventing the progression of human atherosclerosis driven by autoimmune T cells. These therapies could potentially reduce the burden of cardiovascular disease on public health.
How to reuse
The CC BY 4.0 license requires re-users to give due credit to the creator. It allows re-users to distribute, remix, adapt, and build upon the material in any medium or format, even for commercial purposes.
You can reuse an article (e.g. by copying it to your news site) by adding the following line:
Single-Cell Analysis Supports a Role for T cell Autoimmunity in Atherosclerosis and Helps Pave the Way for New Immunotherapies © 2023 by Katrina Woolcock is licensed under Attribution 4.0 International
Or by simply adding:
Article
© 2023 by Katrina Woolcock / CC BY
To learn more about the available options, and for details, please consult New Ground’s How to reuse section.
This article – but not the graphics or images – is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
This article – but not the graphics or images – is licensed under a Creative Commons Attribution 4.0 License.
